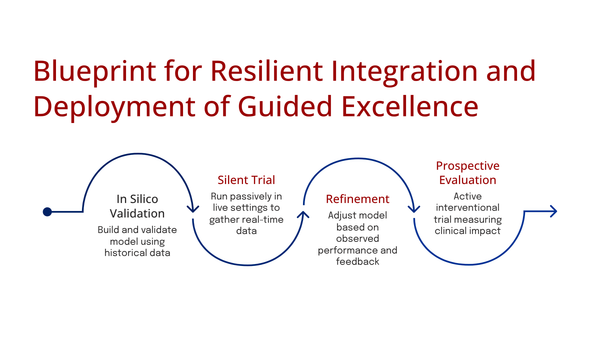
AI-Driven Automation in Mammography
AI could revolutionize mammography by performing initial reads, autonomously clearing a large percentage of normal cases.
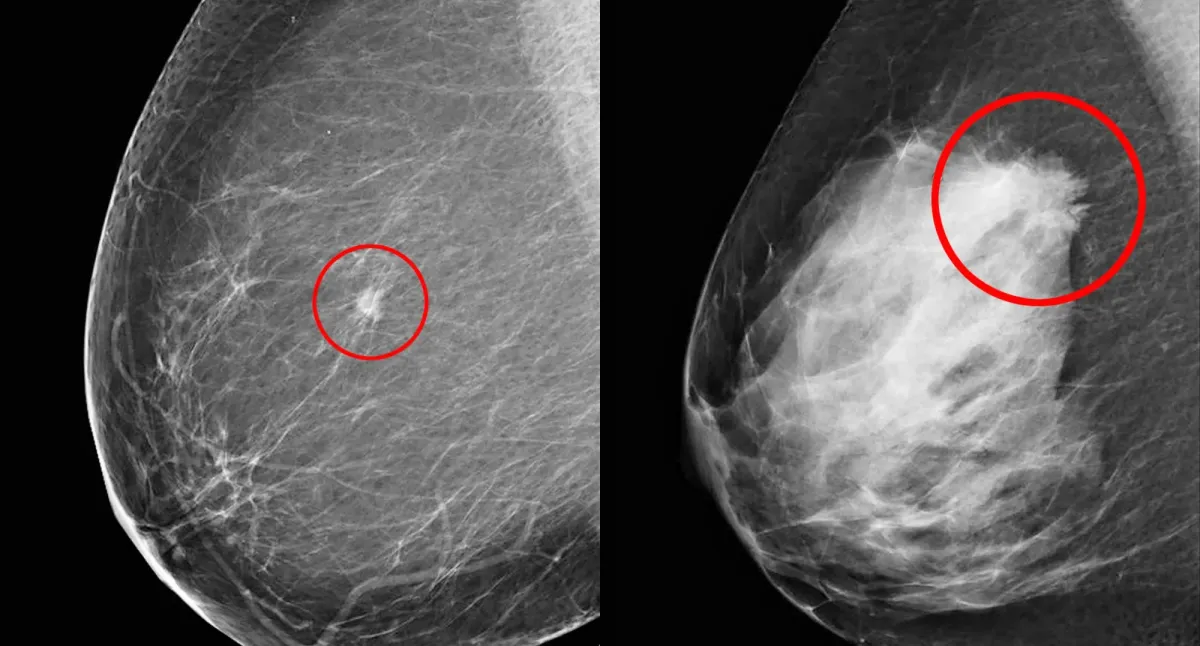
The Origins of Mammography
Mammography originated in 1913 with Albert Salomon's radiography studies of mastectomy specimens (Uddin & Muthalib, 2022). It became a viable clinical tool in the 1960s after Robert L. Egan developed a reproducible technique (Uddin & Muthalib, 2022) and by the 1970s, evidence of its mortality reduction through early detection made it standard for breast cancer screening (Feig, 2014). The 21st century brought a paradigm shift with the FDA's 2000 approval of full-field digital mammography (FFDM), which replaced film with a digital detector for electronic image capture and display (Uddin & Muthalib, 2022; Suleiman, 1999).
Modern Digital Breasts Tomosynthesis
Digital Breast Tomosynthesis (DBT), or 3D mammography, received FDA approval in 2011 for screening (U.S. Food & Drug Administration, 2011). DBT addresses the limitation of overlapping breast tissue in 2D mammography by acquiring a series of low-dose X-ray images from different angles. These images are then reconstructed into thin, high-resolution slices, typically 1 mm thick (Sechopoulos, 2013), allowing radiologists to scroll through the breast tissue layer by layer. This significantly reduces confusion from superimposed structures, a primary cause of missed cancers and false-positive recalls, especially in women with dense breasts (Suleiman, 1999). Clinical studies have shown DBT increases invasive breast cancer detection while decreasing false-positive recall rates compared to 2D mammography (Friedewald et al., 2014), leading to its widespread adoption.
United States & European - Mammography Workflows
In the U.S., screening mammography involves a single, board-certified radiologist interpreting images, predominantly DBT exams, from a PACS worklist. This process relies on comparing current and prior studies to detect subtle changes (American College of Radiology, 2018). After interpretation, the radiologist assigns a BI-RADS assessment (Category 0-6), and the report is sent to the referring physician and patient, adhering to MQSA (U.S. Food & Drug Administration, 1992). Diagnostic mammography is more dynamic, often requiring real-time radiologist involvement for additional views or immediate ultrasound while the patient waits (American College of Radiology, 2018).
In contrast, many European countries, including Germany, Sweden, and the UK, utilize double reading for population-based screening (Perry et al., 2006). Two qualified radiologists independently interpret each mammogram. If both agree the mammogram is normal, a normal result is issued. If one or both identify a suspicious finding, the case is flagged. Disagreements are sent to a third, more senior radiologist for arbitration to determine if the patient should be recalled (Perry et al., 2006).
Future Mammography Workflows
The PRAIM study, a multi-center observational study in Germany, compared standard double-reading to AI-supported double-reading in mammography screening. Radiologists using the Vara MG AI tool as a concurrent reader achieved a statistically superior breast cancer detection rate (BCDR) of 6.7 per 1,000 women, compared to 5.7 per 1,000 in the control group, representing a 17.6% relative increase (Leibig et al., 2024).
The MASAI trial, a large prospective randomized controlled trial in Sweden, assigned over 80,000 women to either standard double reading or an AI-supported workflow. The Transpara AI system triaged cases, sending high-risk exams for a standard double read and low-risk exams for a single read, with radiologists having access to the AI's concurrent reads. Interim results showed the AI-supported workflow detected 20-29% more cancers and led to a 44% reduction in screen-reading workload without a significant increase in false positives (Lång et al., 2023; Salim et al., 2024).
In a real-world deployment, Denmark's Capital Region implemented an AI-driven workflow in November 2021. A commercially available AI system automatically triages mammograms, sending 70% of lowest-risk cases for a single reading and 30% of higher-risk cases for standard double reading, with the AI also acting as a concurrent reader. A retrospective analysis revealed a 33.5% reduction in radiologist workload, a statistically significant increase in cancer detection rate (from 0.70% to 0.82%), and a significant decrease in the false-positive rate (from 2.39% to 1.63%) (Lauritzen et al., 2023).
Best Practices to Learn From
AI could revolutionize mammography by performing initial reads, autonomously clearing a large percentage of normal cases. The Copenhagen implementation, where AI triages ~70% of cases to a single-read pathway, and PRAIM study simulations suggesting 57% of scans could be autonomously cleared without losing cancer detection, illustrate this potential (Leibig et al., 2024). This would significantly free up radiologist capacity.
The most effective workflow combines AI and human strengths. AI acts as a systematic first-pass reader, preventing subtle findings from being overlooked due to human factors, while the human provides essential contextual and clinical filtering. Radiologists validate AI findings, distinguishing true pathology from clinically insignificant findings, creating a safety net. The PRAIM study showed this system detected 204 cancers that would have otherwise been missed when radiologists and AI disagreed (Leibig et al., 2024).
Radiology training must incorporate "AI literacy," moving beyond simply using AI tools to critically evaluating outputs, understanding capabilities and limitations, and recognizing failure modes like algorithmic bias (Parikh et al., 2019). Training should focus on human-in-the-loop workflows, emphasizing adjudication of complex cases and knowing when to override AI suggestions. Clinicians will also play a new role upstream as "AI trainers," curating and labeling high-quality datasets to improve model performance (Topol, 2019).
References
American College of Radiology. (2018). ACR practice parameter for the performance of screening and diagnostic mammography. https://www.google.com/search?q=https://www.acr.org/-/media/ACR/Files/Practice-Parameters/Screen-Diag-Mam.pdf
Barton, M. B. (2024). Artificial intelligence support for radiologists reading screening mammograms. Nature Medicine, 30(1), 58-59.
Beede, E., Baylor, E., Hersch, F., Iurillo, A., Lavoie, M., Ringel, M., ... & Kretz, A. (2020). A human-centered evaluation of a deep learning system for breast cancer detection. In Proceedings of the 2020 CHI conference on human factors in computing systems (pp. 1-12).
Berns, E. A., Hendrick, R. E., Solari, M., & Hotaling, M. A. (2006). Digital and screen-film mammography: a comparison of acquisition and interpretation times. American Journal of Roentgenology, 187(1), 38-41.
Cole, E. B., & Pisano, E. D. (2009). Digital mammography: a new gold standard?. Applied Radiology, 38(3), 16.
Dang, P. A., Kalra, M. G., Blake, M. A., Saini, S., & Halpern, E. F. (2014). Digital breast tomosynthesis: radiologist learning curve. Radiology, 273(3), 697-705.
European Society of Radiology (ESR). (2021). The future of radiology: The ESR perspective on the European Commission's AI White Paper and the role of AI in medical imaging. Insights into Imaging, 12(1), 1-8.
Feig, S. A. (2014). History of breast imaging. Radiologic Clinics, 52(3), 445-453.
Finlayson, S. G., Bowers, J. D., Ito, J., Zittrain, J. L., Beam, A. L., & Kohane, I. S. (2021). The clinician and dataset shift in artificial intelligence. The New England Journal of Medicine, 385(3), 283.
Friedewald, S. M., Rafferty, E. A., Rose, S. L., Durand, M. A., Plecha, D. M., Greenberg, J. S., ... & Copit, D. S. (2014). Breast cancer screening using tomosynthesis in combination with digital mammography. JAMA, 311(24), 2499-2507.
Ghassemi, M., Oakden-Rayner, L., & Beam, A. L. (2021). The false hope of current approaches to explainable artificial intelligence in health care. The Lancet Digital Health, 3(11), e745-e750.
Jha, S., & Topol, E. J. (2016). Adapting to artificial intelligence: radiologists and pathologists as information specialists. JAMA, 316(22), 2353-2354.
Kelly, C. J., Karthikesalingam, A., Suleyman, M., Corrado, G., & King, D. (2019). Key challenges for delivering clinical impact with artificial intelligence. BMC medicine, 17(1), 1-9.
Kim, H. E., Kim, H. H., Han, B. K., Kim, K. H., Ko, K., Nam, H., ... & Choi, J. S. (2021). Changes in cancer detection and false-positive recall in mammography using artificial intelligence: a retrospective, multireader study. The Lancet Digital Health, 3(3), e138-e148.
Lång, K., Environment, A., Rodriguez-Ruiz, A., Zackrisson, S., Aasma, C., Alphonce, J., ... & Dustler, M. (2023). Artificial intelligence–supported screen reading versus standard double reading in the Mammography Screening with Artificial Intelligence (MASAI) trial: a prospective, population-based, randomised controlled trial. The Lancet Oncology, 24(8), 936-944.
Lauritzen, A. D., Nielsen, M. B., Jørgensen, M. G., Jensen, M. C., Heldmann, S., Peters, S., ... & Vejborg, I. M. (2023). Introduction of artificial intelligence in a real-world breast cancer screening program: a retrospective clinical implementation study. Radiology, 309(1), e231268.
Leibig, C., Stüber, T., Wesp, P., Pahn, G., Mertelmeier, T., Nensa, F., ... & Heindel, W. (2024). AI-supported workflow for the interpretation of screening mammograms: a prospective, real-world, multicentre study. Nature Medicine, 30(1), 170-178.
Matheny, M. E., Thadaney Israni, S., Ahmed, M., & Whicher, D. (Eds.). (2019). Artificial intelligence in health care: The hope, the hype, the promise, the peril. NAM Special Publication.
Mildenberger, P., Eichelberg, M., & Martin, E. (2002). Introduction to the DICOM standard. European radiology, 12(4), 920-927.
NHS. (2023, September 14). NHS to roll out AI in breast cancer screening to detect disease earlier. https://www.google.com/search?q=https://www.england.nhs.uk/2023/09/nhs-to-roll-out-ai-in-breast-cancer-screening-to-detect-disease-earlier/
Oeffinger, K. C., Fontham, E. T., Etzioni, R., Herzig, A., Michaelson, J. S., Shih, Y. C. T., ... & Wender, R. (2015). Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA, 314(15), 1599-1614.
Pace, L. E., & Keating, N. L. (2014). A systematic assessment of benefits and risks to guide breast cancer screening decisions. JAMA, 311(13), 1327-1335.
Parikh, R. B., Teeple, S., & Navathe, A. S. (2019). Addressing bias in artificial intelligence in health care. JAMA, 322(24), 2377-2378.
Perry, N., Broeders, M., de Wolf, C., Törnberg, S., Holland, R., & von Karsa, L. (2006). European guidelines for quality assurance in breast cancer screening and diagnosis. Fourth Edition.
Pisano, E. D., Gatsonis, C., Hendrick, E., Yaffe, M., Baum, J. K., Acharyya, S., ... & D'Orsi, C. J. (2005). Diagnostic performance of digital versus film mammography for breast-cancer screening. New England Journal of Medicine, 353(17), 1773-1783.
Price, W. N., & Cohen, I. G. (2019). Privacy in the age of medical big data. Nature Medicine, 25(1), 37-43.
Royal College of Radiologists. (2023). Clinical radiology UK workforce census report 2022. https://www.google.com/search?q=https://www.rcr.ac.uk/system/files/publication/field_publication_file/clinical-radiology-uk-workforce-census-report-2022.pdf
Salim, M., Wåglund, L., Mohammed, A. A., ... & Lång, K. (2024). External validation of an artificial intelligence-based decision-support tool for risk stratification in the Mammography Screening with Artificial Intelligence (MASAI) trial. The Lancet Digital Health, 6(4), e260-e268.
Sechopoulos, I. (2013). A review of breast tomosynthesis. Part I. The image acquisition process. Medical physics, 40(1), 014301.
Sendak, M. P., Gao, M., Nichols, M., Lin, A., & Balu, S. (2020). Beyond the black box: a review of methods for interpreting machine learning models in clinical applications. NPJ Digital Medicine, 3(1), 1-9.
Strickland, N. H. (2000). PACS (picture archiving and communication systems): a new paradigm for radiology. British Medical Journal, 320(7244), 1234.
Suleiman, O. H. (1999). A century of innovation: a history of medical imaging. Radiology Centennial, 1-13.
Topol, E. J. (2019). Deep medicine: how artificial intelligence can make healthcare human again. Basic Books.
U.S. Food & Drug Administration. (1992). Mammography Quality Standards Act (MQSA). https://www.google.com/search?q=https://www.fda.gov/radiation-emitting-products/mqsa-insights/mammography-quality-standards-act-mqsa
U.S. Food & Drug Administration. (2011, February 11). FDA approves first 3D mammography imaging system. [Press release]. https://www.google.com/search?q=https://wayback.archive-it.org/7993/20170112140411/http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm243292.htm
U.S. Food & Drug Administration. (2021). 510(k) Premarket Notification K211678. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpmn/pmn.cfm?ID=K211678
U.S. Food & Drug Administration. (2023). Predetermined change control plans for artificial intelligence/machine learning (AI/ML)-enabled medical devices: Draft guidance for industry and Food and Drug Administration staff. https://www.google.com/search?q=https://www.fda.gov/regulatory-information/search-fda-guidance-documents/predetermined-change-control-plans-artificial-intelligencemachine-learning-aiml-enabled-medical
U.S. Preventive Services Task Force. (2024). Screening for breast cancer: US Preventive Services Task Force recommendation statement. JAMA, 331(21), 1836-1843.
Uddin, M. S., & Muthalib, A. (2022). Evolution of mammography: a historical review. Journal of Clinical Imaging Science, 12.
UK Research and Innovation. (2024, February 1). National platform to support AI breast cancer screening research. https://www.google.com/search?q=https://www.ukri.org/news/national-platform-to-support-ai-breast-cancer-screening-research/
van der Meijden, A., Rodriguez-Ruiz, A., Le, E. P., ... & Sechopoulos, I. (2024). Randomized controlled trials for AI in medical imaging: a guide for the perplexed. Radiology, 310(3), e232402.
Yaffe, M. J., & Mainprize, J. G. (2011). Risk of radiation-induced breast cancer from mammographic screening. Radiology, 258(1), 98-105.